PPT Chapter 5 Electrons In Atoms PowerPoint Presentation, free
A block of the periodic table is a set of groups of chemical elements whose valence electrons occupy, in the ground state, orbitals that share the same azimuthal quantum number ℓ, i.e. belonging to the same sub-electronic layers.

Periodic Table Of Elements With Orbitals
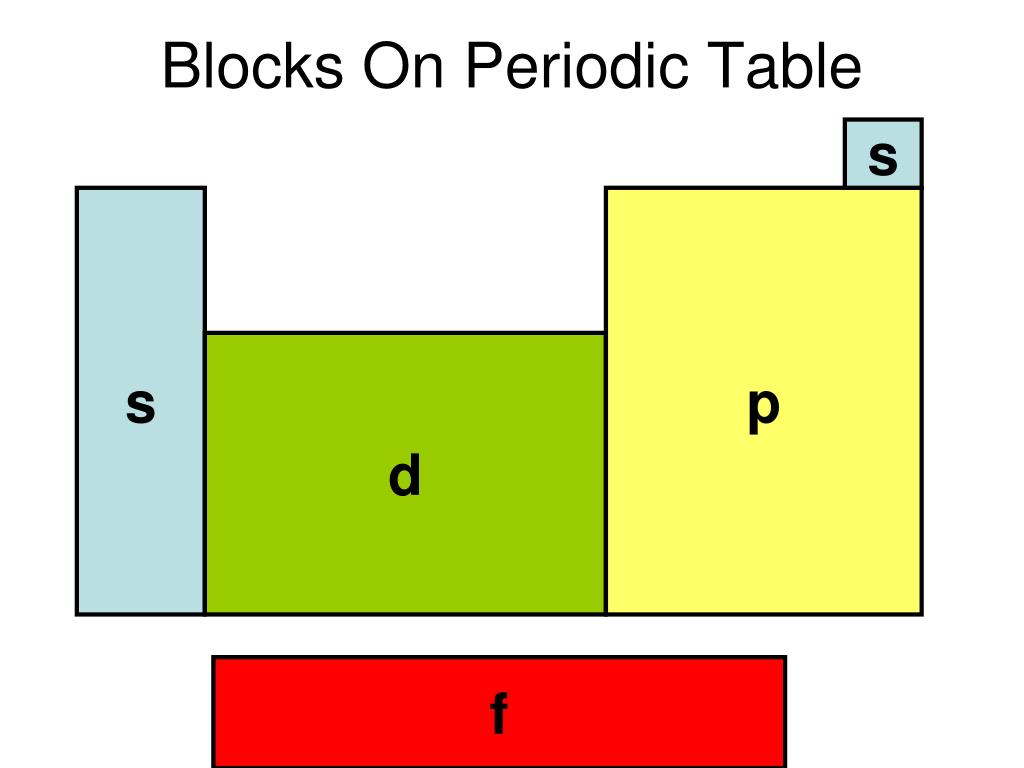
The completely filled d orbitals count as core, not valence, electrons. The two far-left columns comprise the s -block and the six far-right columns constitute the p -block. The noble gases, which are a part of the p -block, all have eight valence electrons except for helium, which has two. These elements are highly stable and unreactive.

Representative Elements Periodic Table
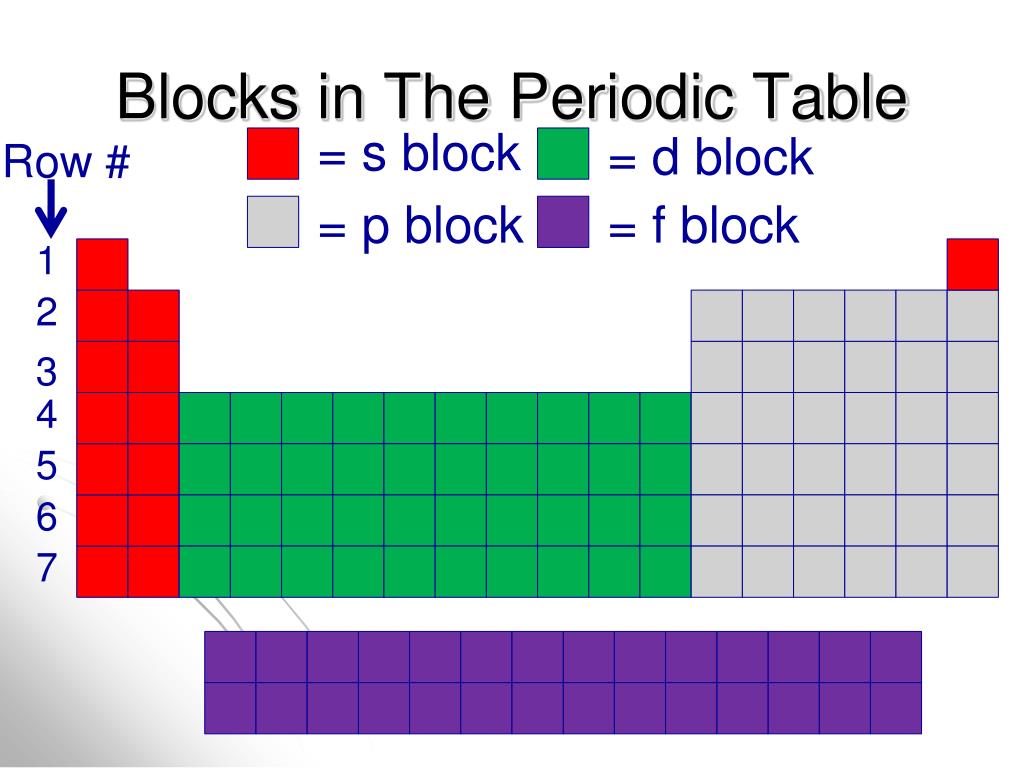
Each orbital can be represented by specific blocks on the periodic table. The s-block is the region of the alkali metals including helium (Groups 1 & 2), the d-block are the transition metals (Groups 3 to 12), the p-block are the main group elements from Groups 13 to 18, and the f-block are the lanthanides and actinides series.

Periodic Table Blocks S P D F Periodic Table Timeline
What Is an Element Block? An element block is a set of elements located in adjacent element groups. Charles Janet first applied the term (in French). The block names (s, p, d, f) originated from descriptions of spectroscopic lines of atomic orbitals: sharp, principal, diffuse, and fundamental.
Periodic Table Blocks S P D F Periodic Table Timeline
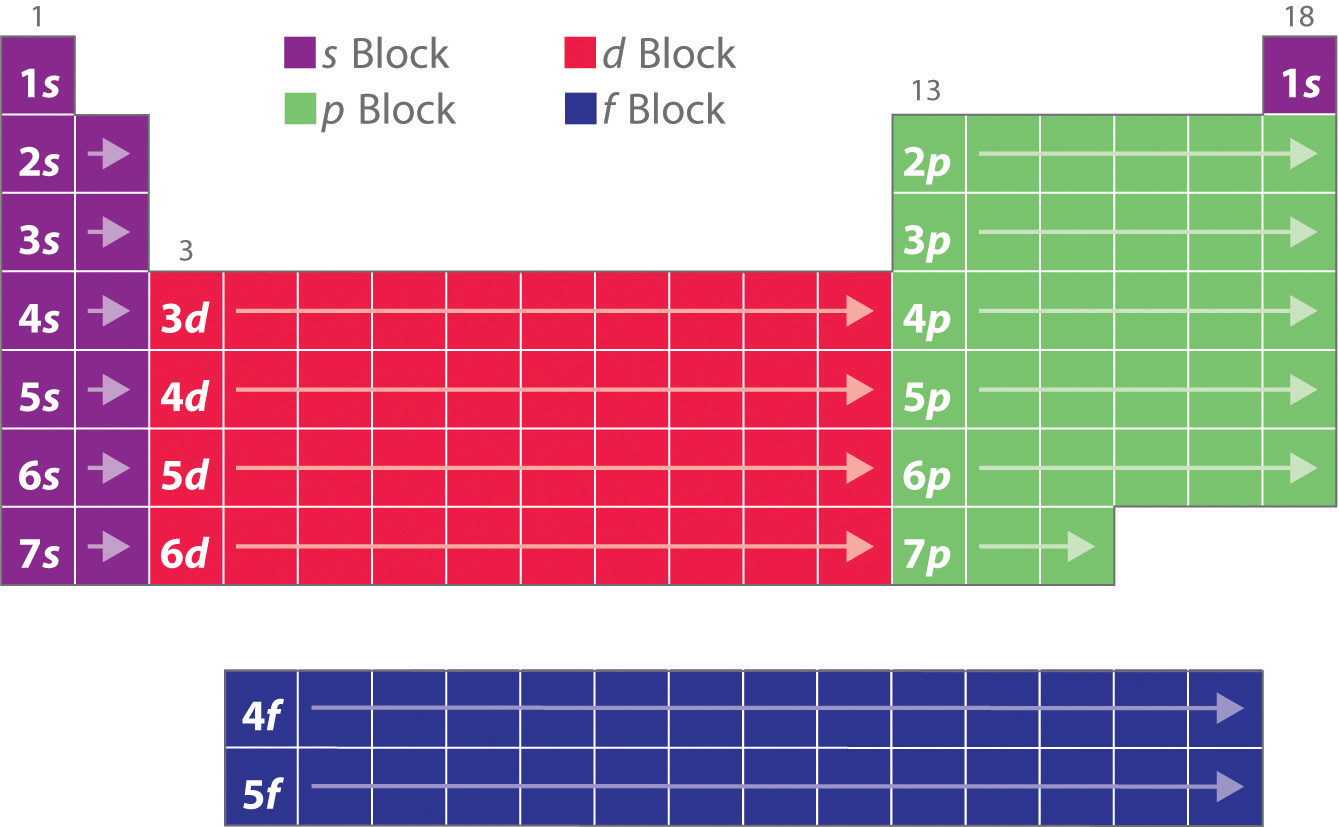
How the periodic table was formed has an intimate correlation with electron configuration. After studying the relationship between electron configuration and the period table, it was pointed out by Niels Bohr that electron configurations are similar for elements within the same group in the periodic table.. S, P, D, and F Blocks. It is easy.

A block diagram of the periodic table shows which sublevels are being
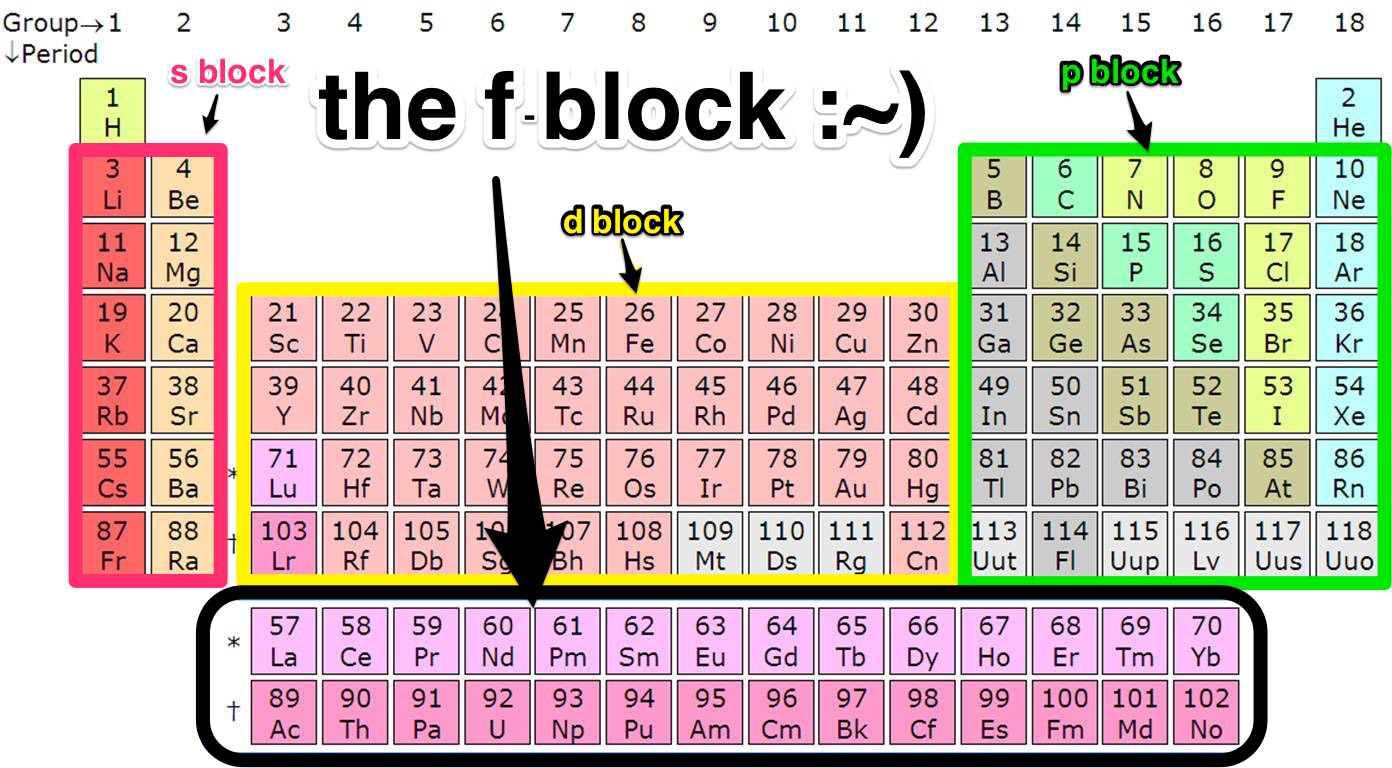
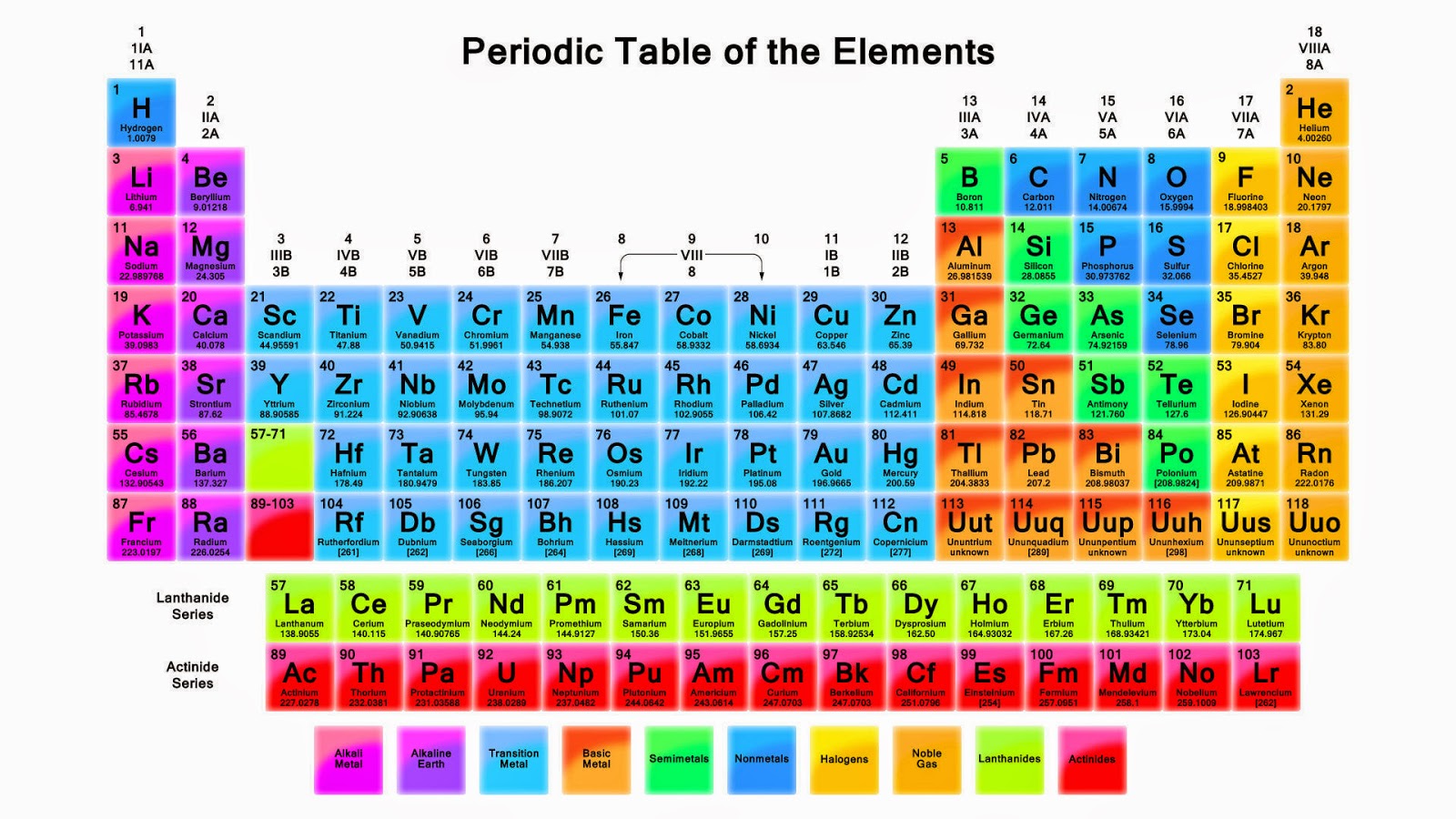
Here are the features 1) Horizontal rows are called period. They were called series in Mendeleev Periodic Table. There are altogether 7 periods in the Periodic table 2) Vertical columns are called groups. Elements have similar outer electronic configuration are arranged in same groups. There are total 18 groups numbered 1 to 18.
The FBlock An introduction » Scienceline
Watch more of this topic at http://bit.ly/1YxZcRFGET MORE CLUTCH!VISIT our website for more of the help you need: http://bit.ly/1YxZhVcSUBSCRIBE for new vi.
3.5 Electronic Structure and the Periodic Table Chemistry LibreTexts
So while there is a possible number of 32 elements in the period, the current number is slightly less. The period to which a given element belongs can be easily determined by its electron configuration. For example, consider the element nickel (Ni) ( Ni). Its electron configuration is [Ar] 3d8 4s2 [ Ar] 3 d 8 4 s 2.

Modern Periodic Table s,p,d,f Blocks Elements Periodic table blocks
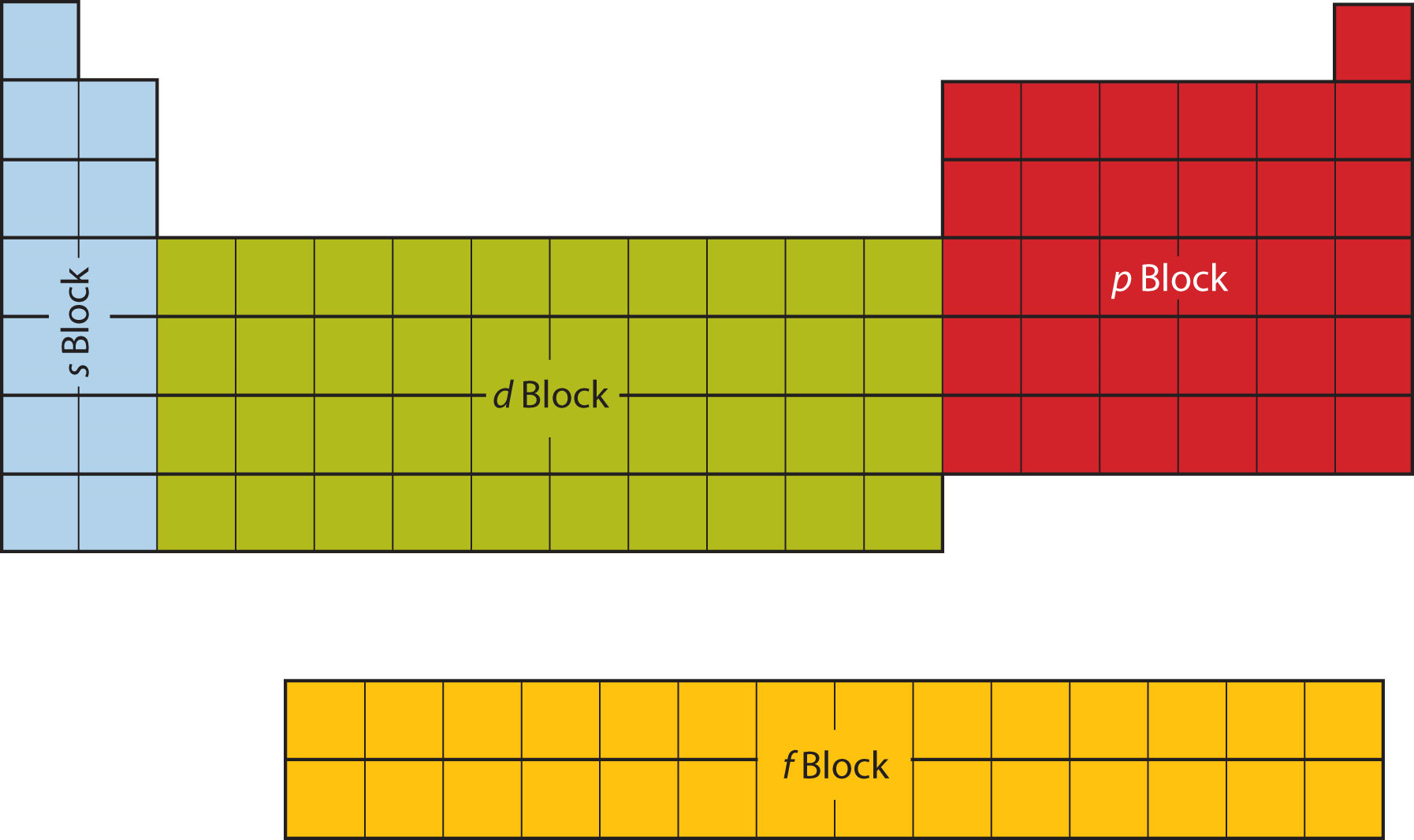
Periodic table blocks are sets of elements grouped by their valence electron orbitals. The four block names are s-block, p-block, d-block, and f-block. Should a new element be discovered, it will be in g-block. Each block indicates which electron sublevel is in the process of being filled.
Periodic Table Split Into Blocks
Steps for Identifying S, P, D, & F -Block Elements. Step 1: Find the element on the periodic table. Step 2: Use periodic table landmarks and mnemonic devices to determine the block.
Periodic Table As Per Blocks
P Block Elements Elements in which the final electron enters one of the three p-orbitals of their respective shells are referred to as P block elements. Because a p-subshell has three degenerate p-orbitals, each of which can accommodate two electrons, there are a total of six groups of p-block elements in a p-subshell.

Download Periodic Table Of Elements S P D F Blocks Online Printable PDF DOC
The periodic table is classified into four blocks based on which subshell the valence electron enters. They are namely s,p,d and f blocks. s Block Elements The s block elements are situated at the extreme left side of the periodic table. They include Group 1 elements (Alkali metals), Group 2 elements (Alkaline earth metals), Hydrogen and Helium.

Periodic Table Blocks S P D F
The periodic table is divided into four blocks, namely s-block, p-block, d-block, and f-block, which correspond to specific regions of the periodic table where electrons are filled in the s, p, d, and f subshells. The s-block elements have their outermost electrons in the s-subshell, while the p-block elements have their outermost electrons in.
Everything's Here sblock Elements and pblock Elements
This block contains the elements of groups 3 to 12 of the periodic table. The three series of transition metals are known 3d series, 4d series and 5d series. f-Block Elements The f-block consists of two series lanthanides and actinides of the periodic table. The electronic configuration of actinides is irregular.

Periodic Table Blocks S P D F Periodic Table Timeline
The periodic table of s‐, p‐, d‐, and f‐block elements. Source publication +4 The Pivotal Role of s‐, p‐, and f‐Block Metals in Water Electrolysis: Status Quo and Perspectives.
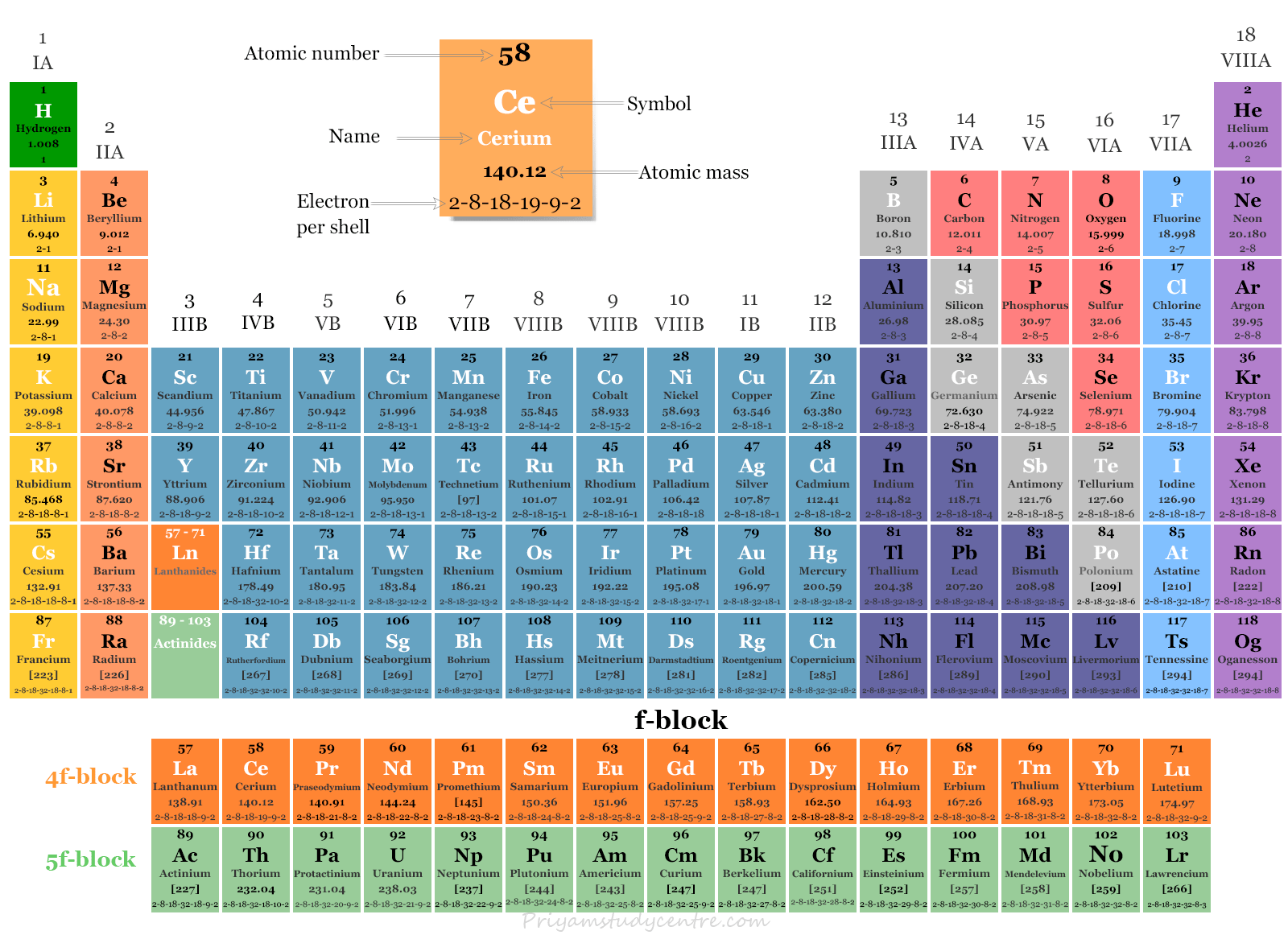
f block Elements Lanthanides and Actinides Periodic Table
These are s, p, d, and f block elements that constitute the whole periodic table. The term block was used by Charles Janet for the first time when he introduced his left step periodic table (LSPT). The divisions into the blocks are characterized by their distinctive nature. For example: